A thermogel is a polymer solution which exhibits the property of transitioning between a liquid and a firm gel during temperature transition. A common-type of thermogel would be one which solidifies upon cooling. An example of this is the ever popular dessert gelatin. For these types of gels, cooling causes polymer chain-entanglement thus forming a solid.
However, for depot-drug-delivery applications, typically we are working with the opposite kind of gel which is one that solidifies upon heating. The benefit of this is that a cold solution can be administered to a patient and then transitions into a solid gel inside the patient. This occurs as the polymer ‘dewaters’ at increased temperatures, transitioning from loose micelles into aggregates that gel together.
This can occur within the lower-critical-solubility temperature where a critical concentration of polymer at the right temperature enables a gel to form.
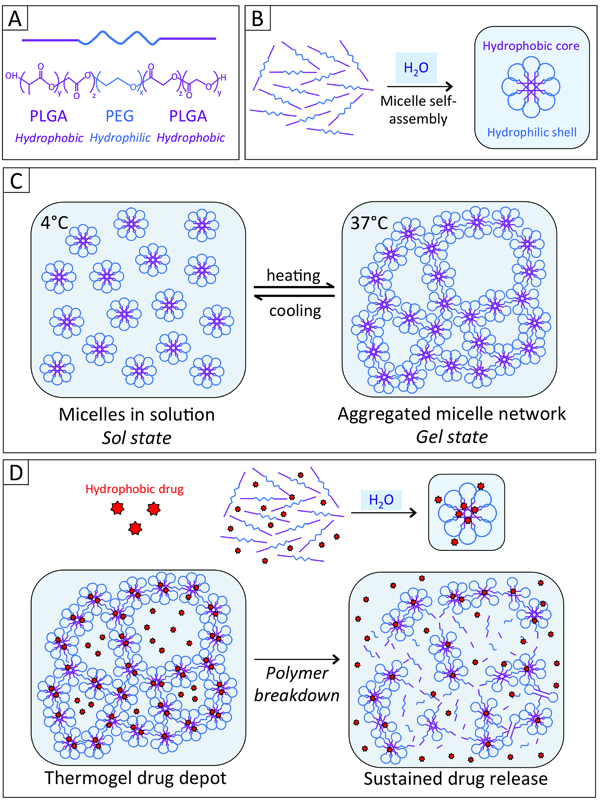
Tri-block thermogel polymer. A. Individual polymer subunit structure. B. When placed in the presence of water, individual polymer subunits self-assemble into micelles with hydrophobic PLGA cores and hydrophilic PEG shells. C. At low temperatures, micelles remain in aqueous solution. As the temperature is raised, cross-link formation between micelles results in formation of a three-dimensional aggregated micelle network D. Hydrophobic drugs can be incorporated into the micelle core and thus solubilized in the polymer solution. Hydrolytic degradation of the polymer network results in slow sustained release of a drug from the micelle cores.
(Image from: Gervais, Kristen J. “Evaluation of a biodegradable thermogel polymer for intraocular delivery of cyclosporine A to prevent posterior capsule opacification.” PhD diss., The Ohio State University, 2017.)
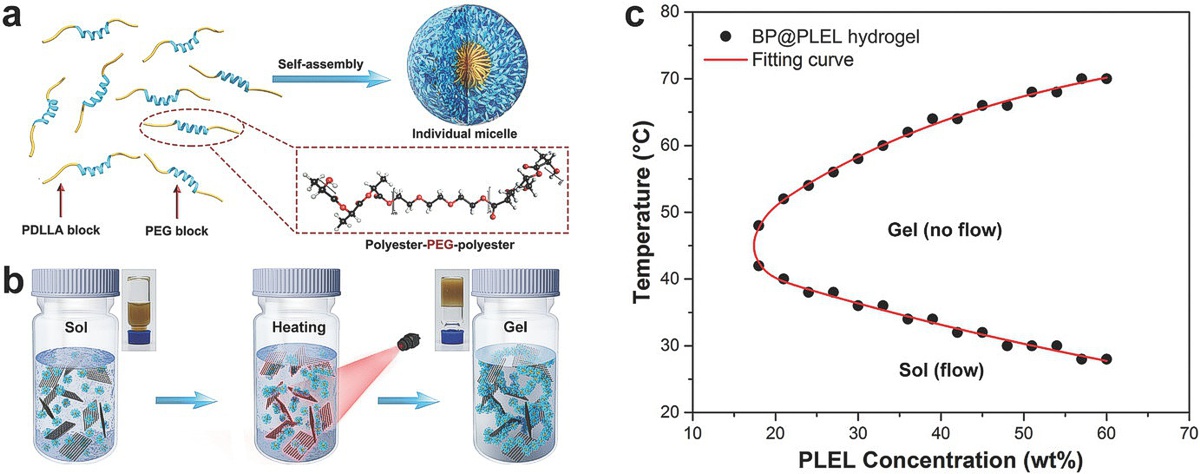
(Image from: Shao, Jundong, Changshun Ruan, Hanhan Xie, Zhibin Li, Huaiyu Wang, Paul K. Chu, and Xue-Feng Yu “Black-Phosphorus-Incorporated Hydrogel as a Sprayable and Biodegradable Photothermal Platform for Postsurgical Treatment of Cancer” Adv. Sci. 2018, 1700848, 3 March 2018, 10.1002/advs.201700848)
It’s worth notingthat is that at high concentrations the polymer is simply insoluble regardless of temperature and at low concentrations it does not display an actual transition into a gel (too dilute). At temperatures above the gel-point, the gel can further dewater to completely collapse into a precipitate completely separated away from the water itself.
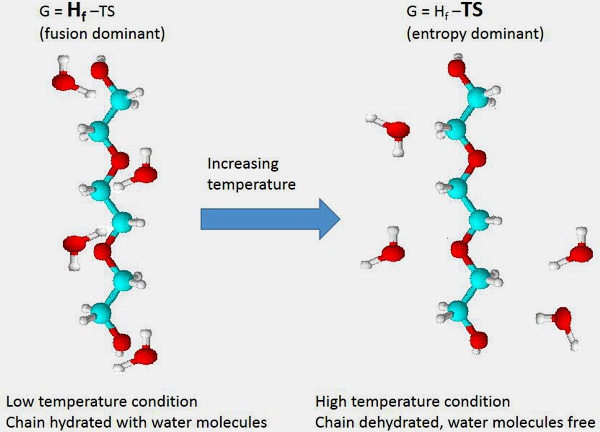
One classic misconception is that upon heating the polymers become hydrophobic. This is not necessarily the case as the polymers have hydrophobic and hydrophilic domains at all times, but the change in temperature affects the relative impact of the organized/disorganized state of water molecules [R. Pelton, “Poly(N-isopropylacrylamide) (PNIPAM) is never hydrophobic.” Journal of Colloid and Interface Science 348(2) (2010) 673-674]. For a good theoretical background as to the driving principles of temperature response, a simple thermally responsive polymer, PEG, can be used as a general model to apply to other systems. PEG by itself displays a cloud point, a higher level temperature at which the polymer precipitates out of water, which varies by molecular weight, but is reported to exist around 100-120 °C. PEG has a unique interaction with water in that when in a dissolved state (or in any state other than freshly dehydrated) each PEG chain monomer is tightly bound to 2-3 water molecules and the water molecules participating in this binding are highly organized leading to a decreased entropy relative to the entropy of free water. The binding energy is an enthalpic term (ΔHf – heat of fusion). As temperature increases the total energy of the system as described as Gibbs free energy of the system (G=H-TS, G-Gibbs free energy, H-enthalpy, T-temperature, S-entropy) favors the entropic term rather than the enthalpic term and the water molecules prefer to be in unorganized form in free solution rather than bound to the PEG chain. J.M. Harris, “Poly (ethylene glycol) chemistry: biotechnical and biomedical applications” Springer, 1992 This process is shown schematically.
WhitePaper: Thermogel Users Guide
WhitePaper: Viscosity and Thermal Properties (AK012)
White-Paper: Thermogels Additive effects and dissolution speed